Biotech Company Given Approval to Reactive Dead Brains with Stem Cells
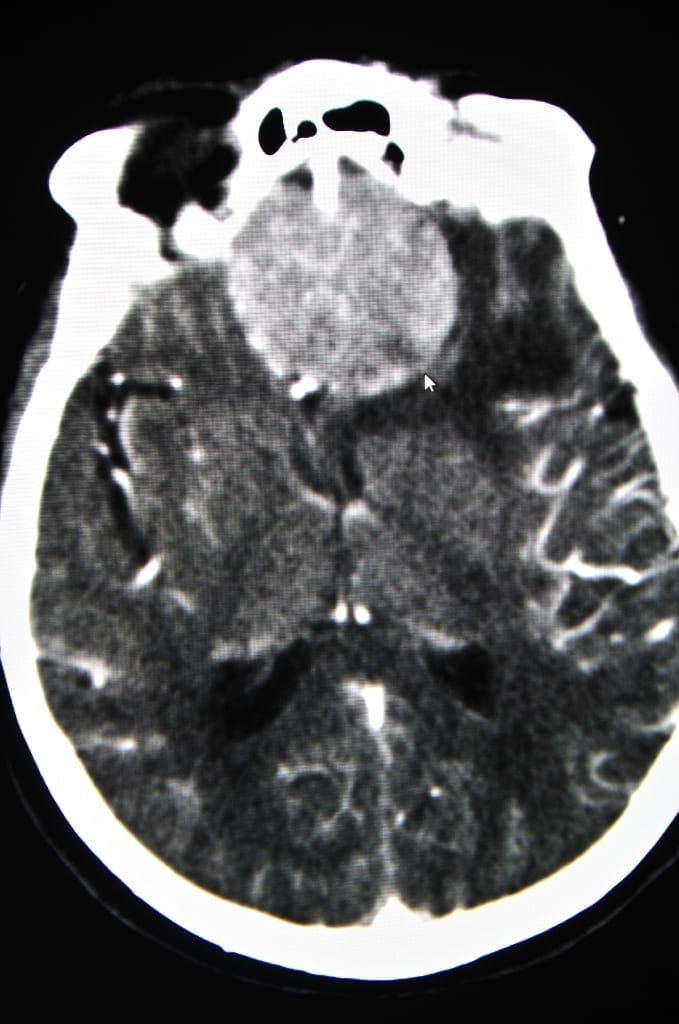
Biotech company Bioquark has been granted ethical permission in the United States and India to use 20 brain-dead patients as part of the groundbreaking ReAnima project.
ReAnima’s scientific research goal is to apply cutting edge tools of regenerative biotechnology to end-stage intensive care scenarios, according to their website. A panel of experts will test a combination of therapies on participants who have been medically certified as brain dead and are only kept from decomposing by life support machines, according to IFLScience.
By injecting the brain with stem cells, giving spinal cord infusions of beneficial chemicals, and utilizing nerve stimulation techniques that have been proven to bring people out of comas, Dr. Calixto Machado and his team will monitor the brain activity of the participants for several months, hoping for signs of neurological reactivation. They will focus on the upper spinal cord, which is the lowermost part of the brain stream that controls a person’s heartbeat and breathing.
“To undertake such a complex initiative, we are combining biologic regenerative medicine tools with other existing medical devices typically used for stimulation of the central nervous system, in patients with other severe disorders of consciousness,” CEO of Bioquark Inc., Ira Pastor, told the Telegraph. “We hope to see results in the first two to three months.”