Computer Simulation of Blood Clots Could Help Treatment
A new computer simulation of blood clots helps to analyze the millions of interactions that happen in a complex chain which is one of the final steps in a complex process with which the human body seals a rupture in an injured blood vessel.
For the full article see below
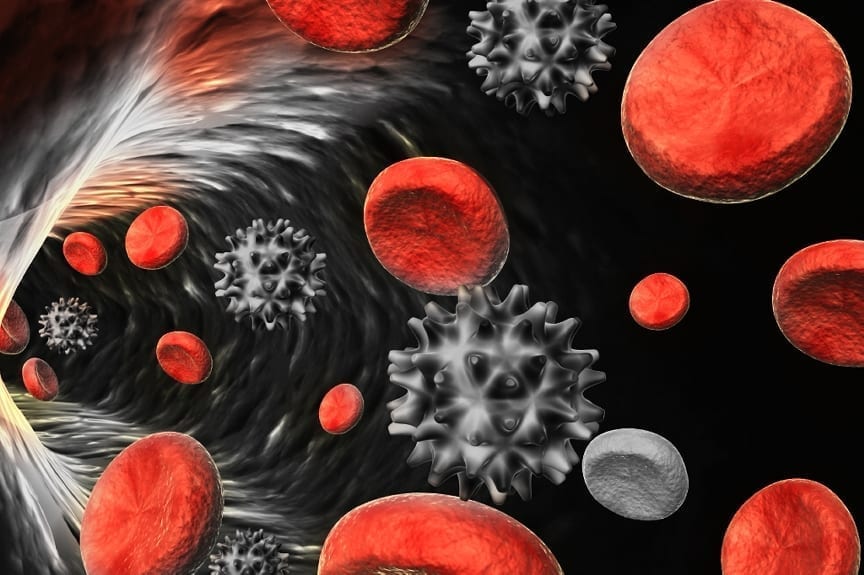
A blood clot is one of the final steps in a complex process with which the human body seals a rupture in an injured blood vessel. Clotting involves interactions between millions of blood cells, microscopic cell fragments called platelets, and various proteins. First, platelets rush to the site of injury and join together with an inner layer of fibrin and collagen proteins to form a sticky web around the break. Red blood cells are then trapped in the web, forming a clot. In certain cases a clot can block arteries and vessels that feed the brain or heart, impeding blood flow and eventually contributing to a stroke or heart attack.
Creating accurate, real-time computer simulations of how blood clots work—and the role they play in medical emergencies—could, in the future, dramatically improve the way that doctors predict the risk of damaging clots and treat the damage incurred by strokes and heart attacks. The models could, for example, help doctors position a stent—a tube placed in a blood vessel to help keep it open—before a risky surgery or offer a new way to test the effects of drugs on the circulatory system. In order to be truly accurate and useful, however, such simulations would have to account for billions of tiny cellular machines, all moving through the blood—something that has never been comprehensively modeled before.
Now, Leopold Grinberg of Brown University and an international team of researchers have used three of the world’s fastest supercomputers to create a detailed and sophisticated model of clot formation in an aneurism—a bulging of a vessel wall as it fills with blood. Grinberg’s team used magnetic resonance imaging (MRI) data taken from a patient with an aneurism to create a realistic model of the major arteries in the patient’s neck and brain and more than 300,000 computer processors to simulate a rupture of the aneurism, blood flow through the system, and formation of a clot.
The resulting multi-scale simulation enables researchers to zoom in from a macroscale view of arterial blood flow to a microscale view of clot formation in the ruptured aneurism. At the large scale, the simulation shows the effect of expanding and contracting arterial walls on the direction of blood flow and resolves complex features such as recirculation regions and swirling flow. At the microscale, the simulation shows platelet aggregation and reveals small platelet clusters detaching from the clot and floating away down the bloodstream.
Grinberg’s team overcame the computational challenge by breaking down the brain’s circulatory system into a manageable patchwork of macro- and microscale models. At the large end of the spectrum, the model is divided into five regions representing the major arteries of the brain (the cerebral, carotid, vertebral and basilar arteries) and the aneurism. To simulate platelet aggregation at the point of rupture, researchers broke down a three-millimeter cubed area inside the aneurism into thousands of triangular-shaped patches. Each patch is a separate computer simulation in which the speed and direction of platelets and blood cells is computed. Researchers then used a series of statistical algorithms to compute the particles’ velocities as they traveled from one region to another, effectively interfacing the separate macro- and microscale models into a fluid multidimensional simulation.
Current modeling techniques such as light transmission aggregometry, a process where the amount of light passing through a culture of activated platelets is measured to determine the severity of a clot, give doctors an idea of how effective treatments are after administering them, but provide little insight into what might be effective beforehand, says Harvard neurologist Thabele Leslie-Mazwi, who was not involved in the new research. And, currently, the only way doctors can test the efficacy of placing a stent in an artery is to do it and see what happens. Accurate computer simulations offer a new way to test anticlotting medication and could be an alternative to cell cultures.
“Currently, doctors have to make an educated guess as to what might be effective at clearing a clot then they check their theory afterwards,” Leslie-Mazwi says. “The drawback to this is that every patient is different and what might work in one could have life-threatening consequences in another.”
Leslie-Mazwi says Grinberg’s work proves the feasibility of computer simulations that could determine beforehand whether or not specific treatments would have the desired effect. He adds that the technology could have big financial implications in the medical field as well, especially for drug companies. “Drug companies spend billions trying to develop products that end up not making it through clinical testing,” he says. “A multi-scale algorithm could be tailored to show what works and what doesn’t. It would give you the ability to predict your investment.”
Source Article
http://www.scientificamerican.com/article.cfm?id=blood-clots-slideshow