New Approved, Fast-Acting Depression Treatment Could Help Millions
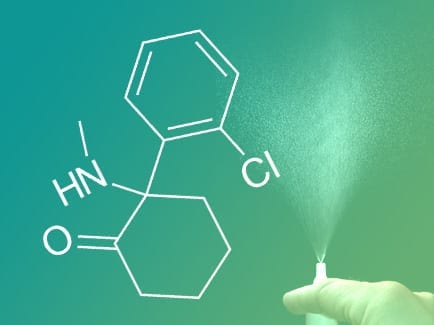
According to the National Institute of Mental Health, over 16 million adults in the US have experienced depression, and many have experienced limited to no success with traditional treatment methods. On Tuesday, however, the Food and Drug Administration (FDA) approved a new prescription treatment that could potentially address this; a nasal spray version of ketamine.
The new treatment, called esketamine, contains an active portion of the “old and widely used anesthetic” ketamine molecule, according to the New York Times. While ketamine’s antidepressant properties aren’t fully understood, hundreds of clinics around the country administer the drug intravenously.
The recommended course of this new treatment, which will be marketed as Spravato, will be administered in a doctor’s office or clinic twice a week for four weeks. Boosters will be administered as needed, and a common oral antidepressant will also be prescribed. Patients should not drive on the day of their treatment and must be monitored for at least two hours after treatment.
Experts claim that “the approval of esketamine marks a new approach to treating serious mood problems.”